Molecules and atoms are extremely small objects - both in size and mass. Consequently, working with them in the laboratory requires a large collection of them. How large does this collection need to be? A standard needs to be introduced. This standard is the "mole". The mole is based upon the carbon-12 isotope. We ask the following question: How many carbon-12 atoms are needed to have a mass of exactly 12 g. That number is NA - Avogadro's number. Thus, NA is defined by Careful measurements yield a value for NA = 6.0221367x10^+23. This is an incredibly large number - almost a trillion trillion. For example, if we stack NA pennies on top of one another how tall would the stack be? The answer is it would be so tall that the stack of pennies could reach the sun and back almost 500 million times! A convenient name is given when there is an Avogadro's number of objects - it is called a "mole". Thus in the above example there was a mole of pennies. The mole concept is no more complicated than the more familiar concept of a dozen : 1 dozen = 12 objects. From the penny example above one might suspect that the mass of a mole of objects is huge. Well, that is true if we're considering a mole of pennies, however a mole of atoms or molecules is a different story. Recall that the atomic mass unit (amu) is defined as 1/12 the mass of a carbon-12 atom. Consequently we have the relation Thus, a mole of carbon-12 atoms has a mass of just 12 g. What about other atoms? In the periodic table the atomic mass of the elements is given. For example the atomic mass of magnesium is 24.305 amu. This is the average isotopic mass of naturally occurring magnesium. What is the molar mass of magnesium in grams? From the equation above we get 1 amu = 1g/NA or 1 amu = 1.66054x10^-24 g. Thus, a mole of magnesium atoms has a mass of NA x 24.305 amu x (1.66054x10^-24 g/amu) = 24.305 g. A mole of magnesium atoms has a mass of 24.305 g. This example demonstrates that the atomic mass of magnesium can be interpreted in one of two ways: (1) the average mass of a single magnesium atom is 24.305 amu or (2) the average mass of a mole of magnesium atoms is 24.305 g; 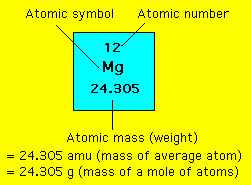 A similar conclusion follows for all of the other elements. |

