The standard for every unit must be defined. Length is an example. The basic unit of length is the meter which was defined in 1983 as equal to the distance traveled by light in a vacuum in 1/299,792,458 of a second. Mass must also be defined. The definition of mass today is the amu (atomic mass unit). The amu is defined in the following way: the mass of one atom of the carbon-12 isotope is EXACTLY 12 amu. All other masses are measured relative to this carbon-12 standard. For example, suppose we do an experiment and find that the isotope bromine-81 has a mass that is 6.743 times that of carbon-12. Then the mass of bromine-81 would be given by 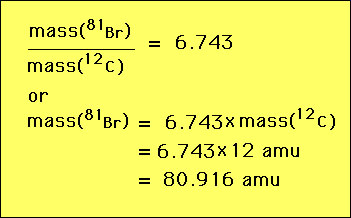 |

