The empirical formula of a molecular compound is the simplest possible formula. The empirical formula contains the correct ratio of atoms in the compound but not necessarily the correct number of atoms. We discussed this earlier. The empirical formula can be obtained from an experimental analysis of the percent composition of the compound. If the mass percent composition of the compound is experimentally determined, we can then use the mole concept to get the empirical formula. For example, consider carboxylic acid. Experimentally it is determined that carboxylic acid contains C(41.4%), O(55.2%)and O. To use this information to determine the empirical formula we first imagine 100 g of carboxylic acid. This 100 g number is convenient because the percentages will then reflect the mass of each element in 100 g of the compound. Therefore we have, in 100 g of carboxylic acid, 41.4 g C, 55.12 g O and 3.48 g of H. This information can then be used along with the atomic mass of the elements to determine the number of moles of each element: 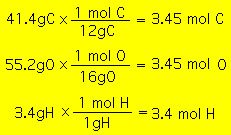 We can then use these mole numbers to get the mole ratios of the elements in carboxylic acid:  And we thus arrive at the empirical formula: It is important NOT to conclude that carboxylic acid molecule has 1 C, 1 H and 1 O. It simply tells us that for every carbon atom there is one hydrogen and one oxygen atom. The formula that tells us the correct number of atoms is called the "molecular" formula. |

