Molecules consist of two or more atoms bonded to one another
through "covalent" bonds. Identification of these molecules
is through their molecular formulas. There is a hierarchy of
formulas:
| Empirical
formula |
Consider the benzene molecule which has 6 carbon and 6 hydrogen
atoms. Therefore the ratio of carbon to hydrogen atoms is 6 to
6 which we can simplify to 1 to 1 (i.e., 1:1). The empirical
formula expresses this most simple ratio, i.e. C1H1 (or CH). The empirical formula expresses
the most simple ratio of atoms in the molecule. Examples:

|
| Molecular
formula |
Molecular formulas go one step beyond the empirical formula
in that they express not only the correct ratio but the correct
number of atoms in the molecule. In the case of benzene the molecular
formula would be C6H6.
other Examples:

Notice that sometimes the empirical and molecular formulas
are the same. This will happen when the molecular formula also
expresses the most simple ratio of atoms.
|
| Structural
formula |
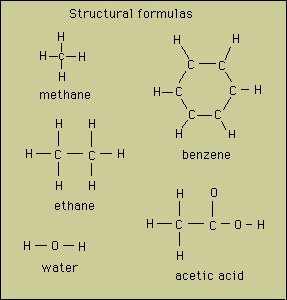
The structural formula not only has the correct number of
atoms but includes the bonding structure of the molecule (i.e.,
which atoms are bonded together). Examples:
|
| 3D
Structural formula |
Molecules are three dimensional (3D) objects. The 3D
structural formula is an attempt to convey the 3D geometry of
the molecule. Examples:
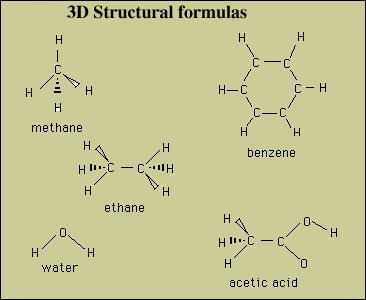
The triangular bonds depict atoms coming out of the plane
and the dashed bonds depict atoms going in back of the plane.
|
| Space
filling formula |
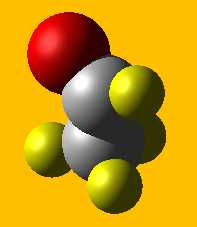
The space filling formula includes the relative sizes
of the atoms. The molecule acetaldehyde (C2H4O) below is an example. Gray spheres are carbon
atoms, yellow spheres are hydrogens and the red sphere is oxygen.
|
| |
|

