|
Charles studied the compressibility of gases nearly a century
after Boyle. In his experiments he observed "At a fixed
pressure, the volume of a gas is proportional to the temperature
of the gas." The experiment is simple: (see figure on left)
A cylinder with a piston and a gas is immersed in a bath (e.g.
water). A mass is placed on top of the piston which results in
a pressure on the gas. This mass is held constant which means
that the pressure on the gas is constant. The gas volume is measured
as the temperature is increased and V vs T data point plotted.
This is continued over a large range of temperatures. To see
what happens place the mouse cursor over the image.
The straight line implies

- which suggests we define a new temperature T (the kelvin
temperature scale) as
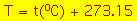
- which leads to

Which is Charle's law (P and n constant)
|

