Atoms have a definite structure. This structure determines the chemical and physical properties of matter. This atomic structure was not fully understood until the discovery of the neutron in 1932. The history of the discovery of atomic structure is one of the most interesting and profound stories in science. In 1910 Rutherford was the first to propose what is accepted today as the basic structure of the atom. Today the Rutherford model is called the "planetary" model of the atom. In the planetary model of the atom there exists a nucleus at the center made up of positively charged particles called "protons" and electrically neutral atoms called "neutrons". Surrounding or "orbiting" this nucleus are the electrons. In elements the number of electrons equals the number of protons. 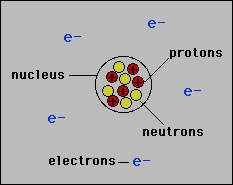 The picture above greatly exaggerates the size of the nucleus relative to that of the atom. The nucleus is about 100,000 times smaller than the atom. Nevertheless, the nucleus contains essentially all of the mass of the atom. In order to discuss the mass of an atom we need to define a new unit of mass appropriate to that of an atom. This new unit of mass is called the "atomic mass unit" or amu. The conversion between the amu and gram is The mass, in amu, of the three particles is given in the table below: 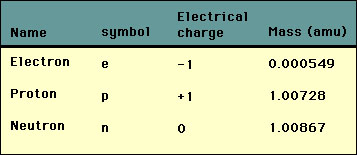 Note that the mass of an electron is about 2000 times smaller than that of the proton and neutron. Also note that the mass of the proton and neutron is close to 1 amu. This is a useful fact to remember. If the number of electrons does not equal the number of protons in the nucleus then the atom is an ion: |

