 |
- Rutherford's Planetary Model
- of the Atom
|
- By 1911 the components of the atom had been discovered. The
atom consisted of subatomic particles called protons and electrons.
However, it was not clear how these protons and electrons were
arranged within the atom. J.J. Thomson suggested the"plum
pudding" model. In this model the electrons and protons
are uniformly mixed throughout the atom:
-

-
- Rutherford tested Thomson's hypothesis by devising his "gold
foil" experiment. Rutherford reasoned that if Thomson's
model was correct then the mass of the atom was spread out throughout
the atom. Then, if he shot high velocity alpha particles (helium
nuclei) at an atom then there would be very little to deflect
the alpha particles. He decided to test this with a thin film
of gold atoms. As expected, most alpha particles went right through
the gold foil but to his amazement a few alpha particles rebounded
almost directly backwards.
-
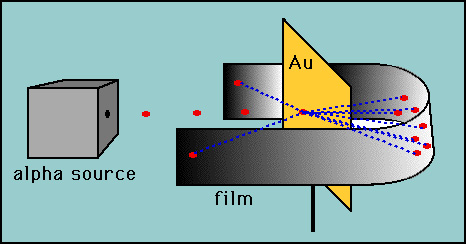
-
- These deflections were not consistent with Thomson's model.
Rutherford was forced to discard the Plum Pudding model and reasoned
that the only way the alpha particles could be deflected backwards
was if most of the mass in an atom was concentrated in a nucleus.
He thus developed the planetary model of the atom which put all
the protons in the nucleus and the electrons orbited around the
nucleus like planets around the sun.
-
-
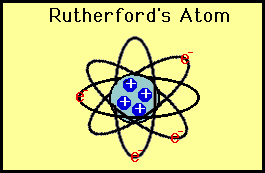
|
|

