 |
- Microscopic/Macroscopic Views
of
- Chemical Reactions
|
For a chemical reaction to take place atoms and molecules
must interact with one another. Atom-atom, atom-molecule or molecule-molecule
collisions must take place. Thinking of the reaction at this
atomic level is the "microscopic" view of the reaction.
On the other hand, when we do experiments with chemical reactions
in the laboratory we see the effects of a very large collection
of atomic and molecular collisions. This latter perspective is
the "macroscopic" view.
- Microscopic view of reactions: single atomic/molecular
collisions
- Macroscopic view of reactions: large collections of
atomic/molecular collisions
Consider the reaction between hydrogen (H2) and oxygen (O2).
From the microscopic view we see the H2 and O2 molecules collide
and exchange atoms. (Place mouse cursor over the image below
to see the animation.)
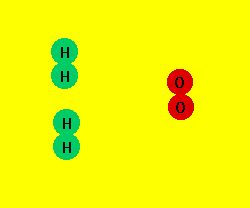
This reaction is, of course, the formation of water. While
the animation above is a simplified version of the microscopic
view, it nevertheless depicts the essence of a chemical reaction
as an exchange or rearrangement of atoms with old bonds breaking
and new bonds forming during the reaction process.
The macroscopic view of the above reaction is different only
in that we are now concerned with a large collection of hydrogen
molecules colliding with oxygen molecules. The number of H2 and
O2 molecules necessary to be seen experimentally is huge - billions
of trillions of individual collisions of these molecules. Later
we will learn that the chemist has a particular name for this
large number - it is called the "mole".
|

