 |
- Balancing Chemical Reactions
|
If we know the chemical formulas for the reactants and products
involved in a chemical reaction, then we can keep track of the
various atoms in the reaction and make sure that atoms are neither
lost or gained in the equation. For example, the combustion og
magnesium metal with oxygen can be written with the formulas
of all reactants and products: Reactants: (magnesium = Mg, oxygen
= O2), Products: Ash = magnesium oxide (MgO). The reaction is
written as
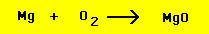
However, a quick examination reveals that, as written, the
equation above has 3 atoms in the reactants and 2 atoms in the
products. This equation cannot therefore obey the conservation
of mass principle. The way we balance it is with "stoichiometric"
coefficients, i.e., numbers in front of the chemicals that denote
how many "items" of that chemical are involved. Thus,
to balance the O2 we put a 2 in front of the MgO. However, now
there are 2 Mg's in the products and only one Mg in the reactants.
To balance the Mg we then put a 2 in front of the Mg in the reactant.
All atoms are now balanced in the reaction (put mouse arrow over
the above reaction to see the result).
Determining the stoichiometric coefficients usually involves
simple inspection of the reaction, as was accomplished above.
However, there are more complex reactions which cannot be balanced
by inspection - a specific balancing procedure must be followed
instead. We will discuss those reactions at the appropriate time.
While balancing reactions atom-for-atom will always work,
sometimes it becomes tedious. However, shortcuts can be taken.
For example consider the reaction of sufuric acid and lead(IV)
hydroxide:

Rather than inspoecting this equation atom by atom, we can
instead inspect it group-by-group: Notice that the SO4-2 (sulfate)
group stays intact. Therefore, rather than look at it as one
sulfur and 4 oxygen atoms, simply balance it as a group. Thus
to balance the 2 sulfate groups in the products put a 2 in fron
to the sulfuric acid. Inspection then tells us that a 4 must
be placed in front of the water (H2O). The final result can be
seen by putting the mouse arrow over the reaction.
|

