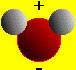
In the section entitled "Chemical Reactions" we discussed precipitation and acid/base reactions within a water-based medium (i.e., aqueous solutions). Water is by far the most important solvent for chemical reactions. A high percentage of naturally occurring chemical reactions occur with water as the solvent. Water has several important properties that make it an interesting solvent: In particular we consider its polarity:
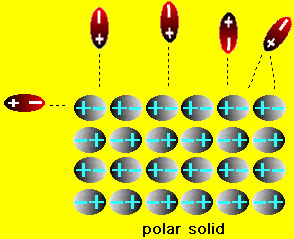
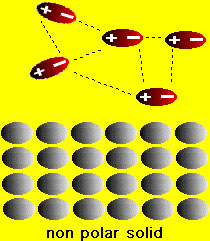
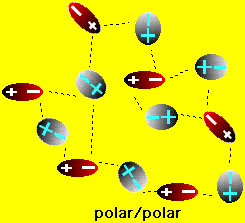
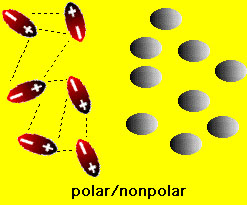
We have already seen how the polarity of water affects the solution of ionic compounds. What happens with aqueous solutions of molecular compounds? Two situations arise in this case: (1) if the molecular compound is a solid (e.g. table sugar (sucrose), vitamin C (ascorbic acid), wax etc.) or (2) the molecular compound is is a liquid (e.g., methanol, benzene, carbon tetrachloride etc.) If the molecules in the solid are polar then it is possible that the solid can dissolve in water. On the other hand if the molecules of the solid are nonpolar then there is no chance that the solid will dissolve in water. This is easy to understand because if the solid has polar molecules then there is an electrostatic attraction between the water and solid molecules. This attraction can lead to solution of the solid (similar to the solution of ionic compounds). On the other hand, if the solid is nonpolar, then water molecules are attract only to one another and basically ignore the solid. consequently the solid does not dissolve. These situations are shown in the figures below
If the molecular compound is a liquid two things can happen when it comes into contact with water: it can either mix with the water or it can separate from the water. When two liquids mix they are said to me "miscible". When two liquids not not mix (separate) they are said to be "immiscible". When will molecular liquids be miscible and when will they be immiscible? The answer is similar to the one for solids above: If the liquid molecules are polar (e.g. methanol (rubbing alcohol)), then it will be miscible with water. If the liquid molecules are nonpolar (e.g., oil), then the liquid will be immiscible with water. These situations are shown in the figures below:
For liquids we come to the conclusion that polar-polar liquids are miscible, polar-nonpolar liquids are immiscible and,as an obvious extension, nonpolar-nonpolar liquids are miscible. This observation leads to the often-said statement "like dissolves like". |