Thus far in all our calculations we assumed that the reaction conditions were ideal and led to reactions that went to 100% completion. Calculation of product mass with these ideal conditions in mind are known as the "theoretical yield". However, in reality reaction are influenced by many "external" factors (i.e., factors apart from the reactants). Some of these factors are:
Depending upon how these various factors, and others, play their role, the reaction, as written, may or may not go to completion. In most cases the reaction does not go to completion. In this case the mass of products formed (the actual yield) is less than the theoretical yield. A quantity that describes this less-than-ideal yield is known as the "percent yield": An example: 50 g of silver nitrate is mixed with 50 g of hydrochloric acid in a water based solution. A white precipitate forms (silver chloride). The solution is filtered and the white precipitate collected and dried. The dried precipitate is measured to have a mass of 53.6 g. What is the theoretical and percent yield? 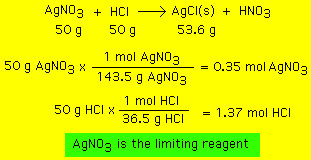 Having identified silver nitrate as the limiting reagent we can do the rest of the calculation:  While the percent yield depends upon the above mentioned factors, it also depends upon the experimenter and their technique. The better an experimenter's technique the higher the percent yield. |

