Characterization of chemical compounds begins with both the elements and their percentages in the compound. The percentage as defined by mass is most directly connected to experiment. The mass percentage of an element in a compound is defined by 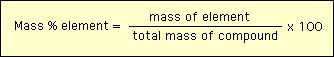 Of course, the sum of the mass percentages of all elements in a conpound must be equal to 100. As an example, consdier the compound methyl ether - which has the molecular formula C2H6O. The mass percentages of C, H and O can be determined in the following way: Consider 1 mole of methyl ether. The mass of 1 mole of methyl ether is equal to 2x12 g + 6x1 g +16 g = 46 g/mol. Then:
These percentages are very important since they are directly related the molecular formula of the compound. |

