Sometimes the empirical formula, which focuses on the correct RATIO of atomic elements in a molecule, also depicts the correct number of atoms in the molecule and sometimes it does not e.g., 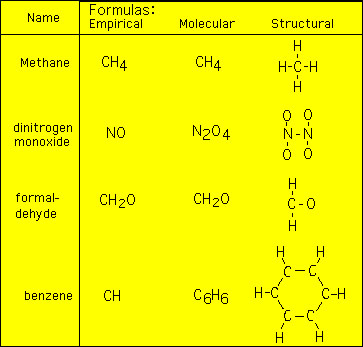 The structural formula is given to visually show the number of atoms in the molecule. Notice that for methane and formaldehyde the structural and molecular formulas are the same. However, for dinitrogen monoxide and benzene the structural and molecular formulas are not. Also note that the molecular formula has the correct number of atoms in the molecule. To get the molecular formula it is necessary to have additional information. Usually, this information takes the form of the molecular weight (MW) of the molecular compound. The procedure is as follows (I'll demonstrate with benzene as the example): 1) Determine the Empirical weight: (for benzene) = 7 2) Get the molecular weight (usually given): (for benzene) = 42 3) Determine the ration of the MW to the EW: (for benzene) = (42/7) = 6 * 4) Multiple the indices for the empirical formula by this
ratio to get the molecular formula: Steps 1-4 will work for all molecules. Try it for dinitrogen monoxide. * This ratio should always result in an integer. |

