|
Light is composed of electric and magnetic fields and thus
its alternate name "electromagnetic spectrum". Light
can be absorbed and emitted by atoms and molecules. The precise
details of which give us the world we see around us. Chemists
are interested in the details of absorption and emission of light
by matter because these details can be viewed as fingerprints
of atoms and molecules as well as their chemical properties.
To understand the interaction of light with matter we need to
first understand the basic character of light
Light is a wave
Traditional physics views light as a
wave - with all the properties usually associated with waves.
For example light can undergo constructive and destructive interference
- just like ripples on a lake.
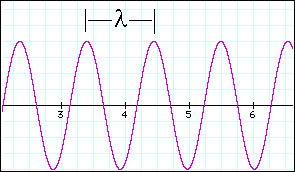
Light is characterized by its
wavelength and frequency
Since light has the characteristics
of a wave then we can associate a wavelength (l) and frequency
(n)
to it: The wavelength is the physical distence between two successive
crests of the wave and the frequency is the number of times the
wave oscillates per second (Hz).
 The frequency and wavelength
of light are related to one another by the speed of light c
The frequency and wavelength
of light are related to one another by the speed of light c
According to Einstein's theory of special relativity nothing
travels faster than light. The speed of light (c) in a vacuum
(e.g., outer space) is 2.998x10+8m/s. The speed of light is related
to the wavelength and frequency by the equation
l n
= c
Since the speed of light does not change, if we know the wavelength
then through this equation we also can determine the frequency
and visa versa.
Light has energy
The energy of light is proportional to its frequency and inversely
proportional to its wavelength. The proportionality constant
is known as Planck's constant h.
Energy = hn
= h c/l
h=6.627x10-34 Joule second
The Spectrum
Light comes in a huge variety of wavelegths.
The visible part of this spectrum (the part that can be seen
with the human eye) is only a very small part of this spectrum.
This spectrum, along with the sources of the radiation, are given
below.
|
l (m)-> |
| 10-12 |
10-10 |
10-8 |
10-7 |
10-6 |
10-5 |
10-2 |
1 |
| |
|
|
|
|
|
|
|
| gamma radiation |
X-rays |
far UV |
UV |
Visible |
IR |
- micro-
- wave
|
radio |
| nucelar events |
innner core
electron |
high E outer
elelctron |
outer electron |
low E outer electrons |
molecular vibrations |
molecular rotations |
transmitters |
|

