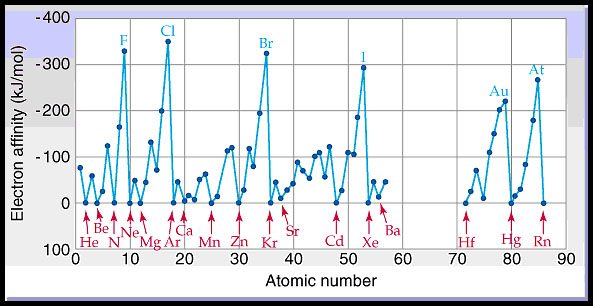
|
Electron affinity is, essentially the opposite of the ionization
energy: Instead of removing an electron from the element we add
an electron to the element to create an anion.
Generally, the energy that results from this process (the
electron affinity) is negative or close to zero. The more negative
this energy the more this process is favored. In the figure below
we see the trends in the electron affinity for many of the elements.
|