|
Photo Electric
Effect
The world of science did not pay too
much attention to Planck's work because his assumption of quanta
was ridiculous to their minds. In 1905 Einstein investigated
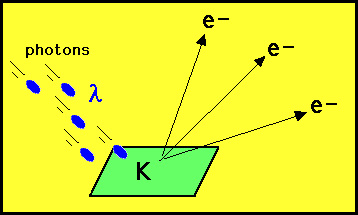
the phenomenon known as the photoelectric effect. The photoelectric
effect is simply the ability of some metals such as potassium
to eject electrons when irradiated by light:
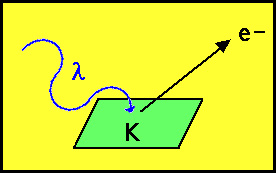
Traditional physics predicted that the
energy of the ejected electron would depend upon the intensity
of light and independent of the wavelength. However, experimentally
the opposite was observed.
Einstein knew of the work of Planck
and decided that it might have application to the photoelectric
effect. Einstein suggested that light, although traditionally
viewed as a wave could instead be viewed as packets of light
he called "photons". These light packets each had energy
which was given by
E(photon) = h n
With this view Einstein was able to
completely explain the photoelectric effect. Einstein clearly
understood that his photons were similar to Plancks' quanta.
|

