 |
- The Aufbau Principle
|
The equations of modern atomic theory are difficult to solve.
Fortunately, many of the results can be obatined by following
some simple rules. These rules are known as the Aufbau principle.
However, we first need to discuss quantum numbers, shells, subshells
and orbitals.
|
The principal quantum number
n - the shell
Quantum numbers abound in quantum theory. These quantum numbers
serve the purpose of keeping track of the various quantum possibilities
that emerge. Perhaps the most important quantum number is the
"principal" quantum number n. The principal quantum
number n can take on the values 1, 2, 3, 4, 5, 6, ... . Associated
with each n is a principle energy level known as a shell. Thus,
shell 1 has n=1, shell 2 has n=2 etc. and so on associated with
it.
|
-
|
Each shell has subshells associated
with it
Depending upon its quantum number, each shell can have one
or more subshells associated with it. For the n=1 shell there
is only one subshell - the s subshell. For the n=2 shell there
are two subshells - the s and p subshells and so on. The number
of subshells within a shell is equal to n.
| principle
quantum number |
number
of subshells |
the subshell
labels |
| 1 |
1 |
s |
| 2 |
2 |
s, p |
| 3 |
3 |
s, p, d |
| 4 |
4 |
s, p, d, f |
|
The shells, subshells and orbitals
can be summarized with the diagram below for a typical atom.
(A mnemonic device exists to recall this order.)
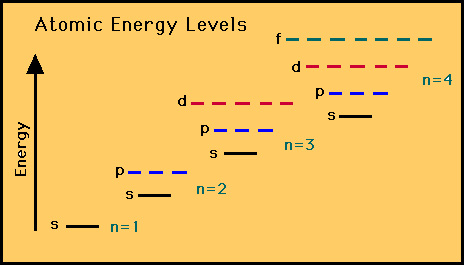
|
|
Each subshell has one or more
orbitals within it
Orbitals are like "rooms" within which electrons
"reside". The s subshell has one s-orbital. The p subshell
has three p-orbitals. The possibilities are listed in the table
below:
|
subshell |
- type of
- orbital
|
- number of
- orbitals
|
| s |
s |
1 |
| p |
p |
3 |
| d |
d |
5 |
| f |
f |
7 |
| g |
g |
9 |
|
|
The Aufbau Principle
The physical and chemical properties
of elements is determined by the atomic structure. The atomic
structure is, in turn, determined by the electrons and which
shells, subshells and orbitals they reside in. The rules af placing
electrons within shells is known as the Aufbau principle. These
rules are:
| 1. |
Electrons are placed in the lowest
energetically available subshell. |
| 2. |
An orbital can hold at most 2 electrons. |
| 3. |
If two or more energetically equivalent
orbitals are available (e.g., p, d etc.) then electrons should
be spread out before they are paired up (Hund's rule). |
Examples
|
|


