 |
- The Bohr Atom
|
- In 1913 Niels Bohr came to work in the laboratory of Ernest
Rutherford. Rutherford, who had a few years earlier, discovered
the planetary model of the atom asked Bohr to work on it because
there were some problems with the model: According to the physics
of the time, Rutherford's planetary atom should have an extremely
short lifetime. Bohr thought about the problem and knew of the
emission spectrum of hydrogen. He quickly realized that the two
problems were connected and after some thought came up with the
Bohr model of the atom. Bohr's model of the atom revolutionized
atomic physics.
-
- The Bohr model consists of
four principles:
| 1) |
Electrons assume only
certain orbits around the nucleus. These orbits are stable and
called "stationary" orbits. |
| 2) |
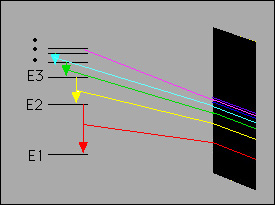
Each orbit has an energy
associated with it. For example the orbit closest to the nucleus
has an energy E1, the next closest E2 and so on. |
| 3) |
Light is emitted
when an electron jumps from a higher orbit to a lower orbit and
absorbed when it jumps from a lower
to higher orbit. |
| 4) |
The energy and frequency of light emitted or absorbed is given
by the difference between the two orbit energies, e.g.,
E(light) = Ef - Ei
n =
E(light)/h
h= Planck's constant = 6.627x10-34
Js
where "f" and "i" represent final and
initial orbits.
|
-
- With these conditions Bohr was able to explain the stability
of atoms as well as the emission spectrum of hydrogen. According
to Bohr's model only certain orbits were allowed which means
only certain energies are possible. These energies naturally
lead to the explanation of the hydrogen atom spectrum:
- Bohr's model was so successful that he immediately
received world-wide fame. Unfortunately, Bohr's model worked
only for hydrogen. Thus the final atomic model was yet to be
developed.
|

