 |
Units
|
Measurement is at the heart of science. Without measurements
to support or reject a hypothesis, science is simply conjecture
- no better than crsytal balls and magic potions. many things
can be measured: Consider any object, we can measure its
-
- and so on. The measurement of most quantities have units
attached to them. howeverm the matter is compicated by the fact
that several units can describe the same quantity, e.g., we can
measure length by inches, centimeters, meters, yards, kilometers,
miles etc. in order to aviod world-wide confusion, scientists
decided to settle on a certain set of units to express basic
quantities - the so-called fundamental units
-
- The Fundamental Units
|
Mass - kilogram (kg)
Length - meter (m)
Time - second (s)
Temperature - Kelvin (K)
Charge - Coulomb (C)
|
-
- All other units are combinations
of these units and are called "derived" units.
-
|
quantity |
unit |
SI |
| speed |
length |
m/s |
| volume |
length+3 |
m+3 |
| energy |
mass length2/time2 |
kg m2/s2 |
| density |
mass/volume |
kg/m3 |
| force |
mass length/time2 |
kg m/s2 |
| pressure |
mass/length time2 |
kg/m s2 |
-
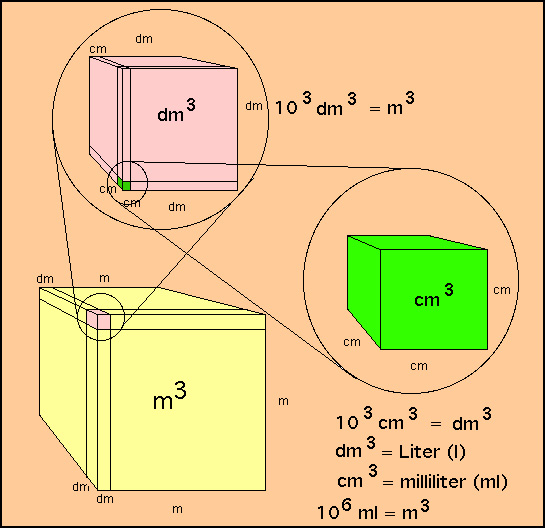
- Volume is an especially important
quantity since it can be readily measured. Volume always has
the units of a cubic length. the relationship between m3, dl
and ml (cc) is readily understood from the diagram below
|

