Specific heat is another physical property of matter. All matter has a temperature associated with it. The temperature of matter is a direct measure of the motion of the molecules: The greater the motion the higher the temperature:  Motion requires energy: The more energy matter has the higher temperature it will also have. Typicall this energy is supplied by heat. Heat loss or gain by matter is equivalent energy loss or gain. With the observation above understood we con now ask the following question: by how much will the temperature of an object increase or decrease by the gain or loss of heat energy? The answer is given by the specific heat (S) of the object. The specific heat of an object is defined in the following way: Take an object of mass m, put in x amount of heat and carefully note the temperature rise, then S is given by  In this definition mass is usually in either grams or kilograms and temperatture is either in kelvin or degres Celcius. Note that the specific heat is "per unit mass". Thus, the specific heat of a gallon of milk is equal to the specific heat of a quart of milk. A related quantity is called the heat capacity (C). of an object. The relation between S and C is C = (mass of obect) x (specific heat of object). A table of some common specific heats and heat capacities is given below:
Consider the specific heat of copper , 0.385 J/g 0C. What this means is that it takes 0.385 Joules of heat to raise 1 gram of copper 1 degree celcius. Thus, if we take 1 gram of copper at 25 0C and add 1 Joule of heat to it, we will find that the temperature of the copper will have risen to 26 0C. We can then ask: How much heat wil it take to raise by 1 0C 2g of copper?. Clearly the answer is 0.385 J for each gram or 2x0.385 J = 0.770 J. What about a pound of copper? A simple way of dealing with different masses of matter is to dtermine the heat capacity C as defined above. Note that C depends upon the size of the object as opposed to S that does not. We are not in position to do some calculations with S and C. Example 1: How much energy does it take to raise the temperature of 50 g of copper by 10 0C? 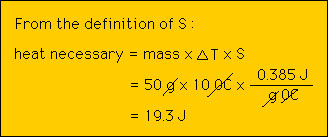 Example 2: If we add 30 J of heat to 10 g of aluminum, by how much will its temperature increase? 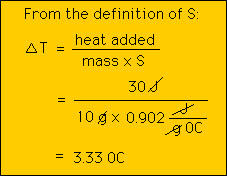 Thus, if the initial temperture of the aluminum was 20 0C then after the heat is added the temperature will be 28.3 0C. |
||||||||||||||||||||||||||||||||||||||

