 |
Conservation of Mass in Chemical
Reactions
|
Democritus (460-370 BC) and somewhat later John Dalton (1766-1844)
were the first to consider matter at its most microscopic form.
They both came up with the concept of the "atom" as
being the smallest unit of matter and thus being undivisible*.
This observation has an important and fundamental consequence:
mass is neither created nor destroyed during the course of
a chemical reaction. How do we come to this conclusion? We
know that chemical reactions take place at the atomic/molecular
level. That is molecules and atoms interact with one onother
during a chemical reaction. If atoms are indivisible then they
cannot be destroyed during a chemical reaction. If atoms cannot
be destroyed then the mass of reactants must equal the mass of
the products in a chemical reaction. e.g.,
|
Reactants -------> Products
Mass of Reactants = Mass of Products
|
This can be visualized by considering the formation of water
from oxygen and hydrogen molecules:
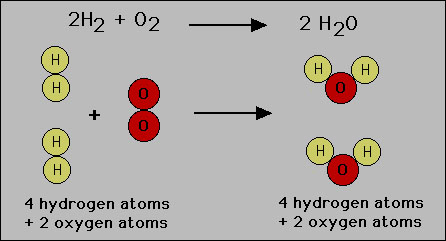
Note that the hydrogen and oxygen atoms simply rearrange themselves
but are not destroyed. Thereofore mass is conserved. Mass conservation
can be used in chemical calculations. For example iron rust by
combining with oxygen to form rust (iron oxide). Suppose 100
g of iron metal rusts. We weigh the rust and find that the rust
has a mass of 143 g. What mass of oxygen reacted with the iron?
|
Iron + Oxygen -----> Rust
100 g + ?g ------> 143g
mass reactants = mass products
mass products = 143g = mass reactants
= 100 + mass of oxygen
mass oxygen = 43 g
|
|


