When matter undergoes transformations that change its chemical and physical properties then that transformation was brought about by a chemical reaction. On the other hand chemical reactions can only take place if there is sufficient energy to make the reaction proceed. Therefore energy is a prerequisite for chemical reactions. Energy can come in many forms e.g., heat, work, light, kinetic, potential, chemical etc.. Moreover, energy can itself transform among these various forms. For example a ball at the edge of a table has zero kinetic energy and positive potential energy. If the ball drops it will have zero portential energy and positive kinetic energy the instant it hits the floor. However the sum of the potential abnd kinetic energy is the same throughout the ball's dropping history. Therefore energy has neither been created or destroyed but has transformed from potential to kinetic energy. 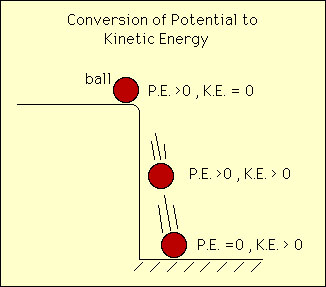 Molecular of chemical energy can mean several things: Chemical bonds are a source of energy, the movement of molecules in space is kinetic energy, the vibrations and rotations of molecules is another soource of chemical energy. All of these forms of chemical energy contribute in one way or another to chemical reactions. The units of chemical reactions are straightforward and is given in the diagram below: 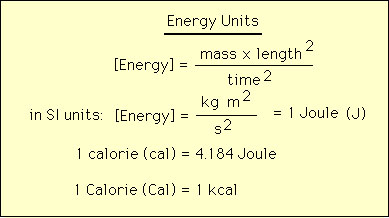 There are many other units for energy including electron volt (ev), erg, kjoule (kJ) etc. |

