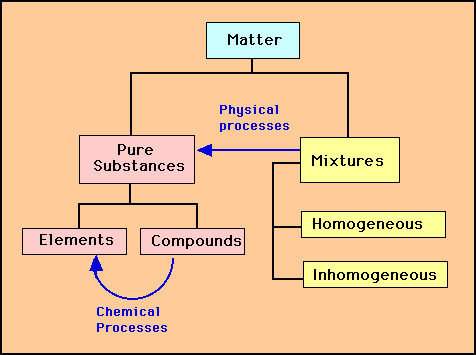
All substances have mass and therefore must be composed of atoms. These atoms and how they assemble themselves in the substance determines their chemical and physical properties. Substances can be classified according to how these atoms are assembled and is known as Classification of Matter: All matter falls into one of three categroies: elements, compounds or mixtures. Furthermore, mixtures can be classified as homogeneous or inhomogeneous. The scheme looks something like the diagram below:
This classification depends upon how we try and separate matter into its basic components. This separation is called the "process". There are two processes: a physical and a chemical process.
If we have a sample of matter and can find a physical process such as evaporation, magnets, color etc. to separate it then the sample is a "mixture". Furthermore if the sample is a mixture of solids and liquids (e.g., sand and water) etc. or two or more liquids that don't mix (e.g., oil and vinigar) then the mixture is "inhomogeneous". Otherwise the sample is a "homogeneous" mixture. If there is no physical process that will separate the sample then the sample is a "pure" substance. If a chemical process such as combustion or oxidation breaks the substance down to its constituent atoms then the substance is a "compound"(e.g., salt, sugar, water). Otherwise the substance is an "element" (e.g., copper penny, aluminum foil). Compounds are made up of molecules or salts. elements are made up of single types of atoms. |