Density is a physical property of matter. Most commonly density referes either to the mass per unit volume (mass density) or the number of objects (e.g., atoms, molecules) per unit volume (number density). We will focus out attention on mass density. The mass density has the units mass/volume. Since volume has the units length "cubed" then the SI unit of mass density is kg/m3. More common units of density are g/ml or g/l. Substances have differnt densities. In fact the density of a substance can often be used to help idenify it. Below is a table of densities of common materials:
An important example is water. The above table state that liquid water has a mass of 1 g in every ml. Thus 2 ml of water has a mass of 2 g etc.. Table sugar is more dense than water by about 60 percent. Density does not depend upon size. For example the water in a swimming pool has the same density a glass of that swimming pool water. Calculations with density are straighforward and involve the formula for density namely D=m/V, where D=density, m= mass and V = volume. Example 1: What is the volume of a nugget of gold that has a mass of 3.45 g? The density of gold can be looked upon as a conversion factor from mass to volume i.e., 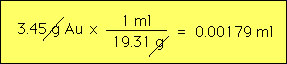 Example 2: A light substance is found to weigh 23 g and to have a volume of 0.192 liters. What is the substance?  Based upon this result we would guess that this substance might be balsa wood. Eample 3: What is the mass of 1 liter of sugar? 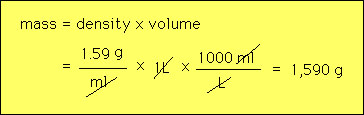 |
||||||||||||||||||||||||||

