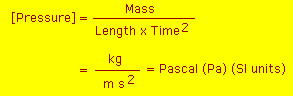|
Consider gas molecules in a rectangular box. Every time a
molecule collides with a wall of the box the collision results
in a force on the box. These forces combine and result in the
pressure of the gas.
Units of Pressure
As shown above the SI unit of pressure is the pascal (Pa).
However, the pascal is not the most convenient unit of pressure.
The table below lists some more common units of pressure.
| Pressure
Unit |
Abbrev. |
Conversion |
| Pascal |
Pa |
--- |
| mm of mercury |
mmHg |
--- |
| atmosphere |
atm |
- 760 mmHg
- =101,325 Pa
|
| bar |
bar |
100,000 Pa |
| Torr |
Torr |
mmHg |
| pound/sq
inch |
lb/in^2 |
51.7 mmHg |
|
Most calculations in chemistry involving gases use the pascal
and the atmosphere. However, coversions between the various units
are straighforward. Example How many pascals is 500 mmHg? Using
the table on the left we find:

|