 |
Isotopes and Atomic Symbols
|
Atomic Symbols:
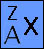 The atom of each element is made
up of electrons, protons and neutrons. All atoms of the same
neutral element have the same number of protons and electrons
but the number of neutrons can differ. Atoms of the same element
but different neutrons are called isotopes. Because of these
isotopes it becomes necessary to develop a notation to distinguish
one isotope from another - the atomic symbol. The atomic symbol
has three parts to it: The atom of each element is made
up of electrons, protons and neutrons. All atoms of the same
neutral element have the same number of protons and electrons
but the number of neutrons can differ. Atoms of the same element
but different neutrons are called isotopes. Because of these
isotopes it becomes necessary to develop a notation to distinguish
one isotope from another - the atomic symbol. The atomic symbol
has three parts to it:
Examples 1:
Consider two isotopes of gallium, one having the 37 neutrons
and the other having 39 neutrons. Write the atomic symbols for
each isotope. Solution:

Example 2:
How many neutrons does the isotope of copper with mass number
Z = 65 have? Solution: From the periodic table we see that copper
has an atomic number of 29. Since Z is the number of protons
plus the number of neutrons, then No. neutrons = 65 - 29 = 36
|


