There are 112 elements although elements 110-112 are as yet unnamed. These 112 elements are organized in the periodic table: 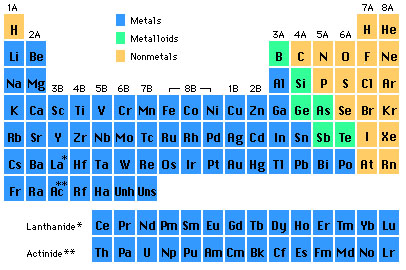 The modern chemical symbols were introduced by Berzelius. Rows of elements are called "periods" and columns of elements are called "groups" (1A, 2A 3B etc.). There are three general classes of elements distinguished by their physical properties: the metals (generally shiny and conduct electricity), the nonmetals (not shiny, sometimes gasses at STP and poor conductors of electricity) and the metalloids (properties in between those of metals and nonmetals.). Some groups have special names:
Many of the heavier elements are unstable - which means that the atoms of those elements break apart very quickly. Elements within a group share similar chemical properties. Other chemical and physical properties of the elements can be deduced from their position in the periodic table. The structure of the periodic table and thus their chemical and physical properties is directly related to their atomic structure.
|

