Molecules can also be charged. Charged molecules are known as polyatomic ions. Since there is a huge variety of molecules there is also the possibility of a huge variety of polyatomic ions. Fortunately there are ony a few common polyatonic ions. Some of the most common are given in the table below: 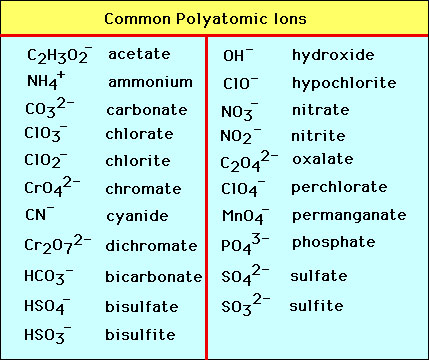 The formulas for the salts of these ions are formed by the usual requirement of electrical neutrality. The name of a salt is the cation name followed by the name of the anion. For example
|

