 |
Elemental Ions and Simple Salts
|
As discussed previously
elemental ions can combine to form salts. These compounds are
known as simple salts. These simple salts are a combination of
metal cations and nonmetal anions. The common ions, their charges
and names are listed below (the names of the cations are the
same as the element, e.g., Ca2+ is the "calcium ion")
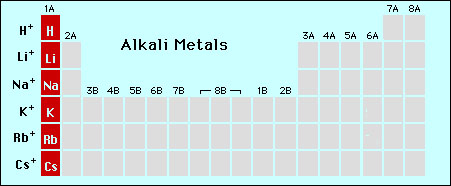 |
Naming simple ionic compounds:
The names of ionic compounds composed of the above ions are
especially simple. The name of the compound is simply the name
of the cation followed by the name of the anion, .e.g, K2S is potassium sulfide. Why is dipotassium
sulfide incorrect? dipotassium sulfide is incorrect because potassium
ion is always K+1 and sulfide is always S-2 and therefore the
only way to combine them is K2S. Therefore
there is a redundancy in the name dipotassium sulfide.
Naming simple ionic compounds with metals that have more
than 1 charge:
Some metals have more than one charge. For example iron (Fe)
has two possible ions, Fe+2 and Fe+3. When this happens the name
of the ion is the element name and, in parenthesis next to it)
a roman numeral denoting the charge. For iron this would be iron(II)
and iron(III).
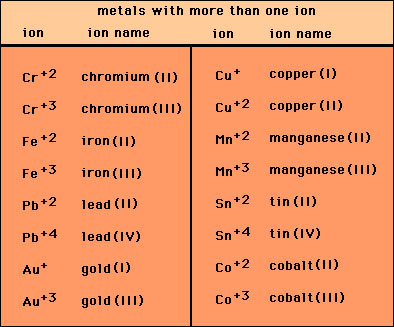
The names of ionic compounds with these ions must include
these roman numerals. For example: FeCl2 has the name iron(II)
chloride, Cr 2S3
has the name chromium(III) sulfide.
|