Consider a simple molecular bond such as that in carbon monoxide (CO). This molecule has 2 nuclei and 6+8 = 14 electrons around these nuclei. The usual Lewis electron-dot structure for CO is (recall that the Lewis structure contains only the valence electrons): Since a single carbon atom has 6 electrons around it and a single oxygen atom has 8 electrons around it, one might expect that in the CO molecule carbon has 6 electrons around it and oxygen has 8 electrons around it. However, that does not turn out to be the case. It turns out that oxygen is slightly better than carbon at "pulling" electrons towards it. Therefore in the CO molecule O has slightly more than 8 electrons around it (giving it a net negative charge) and C (being robbed of this electronic charge by O) has slightly less than 6 electrons giving it a slightly positive charge (equal but opposite to that of the oxygen). The net result is a molecular bond that is electrically "polarized". e.g., ,where the Greek "delta" symbols represent slight positive and negative charges. The ability of an atom in a covalent bond to pull electrons towards it is called its "electronegativity". Atoms can be assigned electronegativities. Generally, the higher the electronegativity the more electronegative the atom. As a general rule, the electronegativities of atoms increase from bottom to top and from left to right (not including the noble gases). Consequently Fluorine is the most electronegative element. 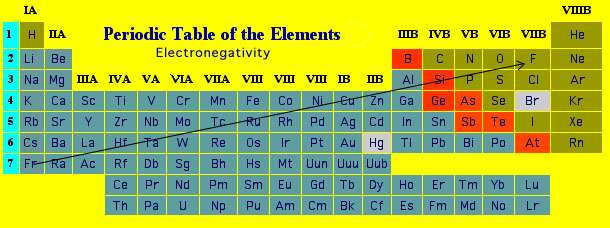 Specific electronegativities can be found using the periodic table link at the top of this and every other page in this website. All molecular bonds between different atoms are polar. Bonds between the same type of atom (e.g., C-C) are not polar since they both have the same electronegativity. The fact that bond can be polar explains a wide variety of physical and chemical phenomena and will be discussed at the appropriate junctures in these notes. |

