 |
- Precipitation Reactions
|
A precipitate is a solid that forms out of solution. A common
example is that of the mixing of two clear solutions: (1) silver
nitrate (AgNO3) and (2) sodium chloride (NaCl): The reaction
is

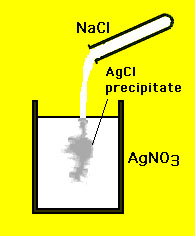 |
The precipitate forms because the
solid (AgCl) is insoluble in water. That is true for all
precipitates - the solids are insoluble in aqueous solutions.
Precipitation reaction occur all around us. For example, sometimes
the pipes in our homes get clogged because precipitates of magnesium
and calcium oxides have deposited themselves within the pipes.
This can happen with "hard" water. Another example
is a kidney stone. A kidney stone is nothing more than a precipitate
- often of calcium ions (from cheese) and oxalates. It is often
suggested that a good way to avoid kidney stones is to drink
a lot of water. This helps because the solubility of the precipitate
increases with the amount of water - thus avoiding the formation
of the kidney stone to begin with. |
|

