Combustion reactions always involve molecular oxygen O2. Anytime anything burns (in the usual sense), it is a combustion reaction. Combustion reactions are almost always exothermic (i.e., they give off heat). For example when wood burns, it must do so in the presence of O2 and a lot of heat is produced:  Wood as well as many common items that combust are organic (i.e., they are made up of carbon, hydrogen and oxygen). When organic molecules combust the reaction products are carbon dioxide and water (as well as heat). 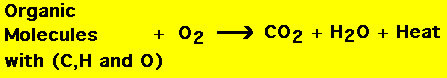 For example consider the combustion of methanol (rubbing alcohol): Of course, not all combustion reactions release CO2 and water, e.g., the combustion of magnesium metal: |