Acid-base reactions are ubiquitous. In aqueous solutions acids increase the hydrogen ion (H+) concentration. On the other hand bases increase the hydroxide ion (OH-) concentration. When an acid and a base react in an aqueous solution the H+ and OH- ions combine to form water. These ions thus "neutralize" one another: Most acids have the general formula HA, where A- is an anion and most bases have the form BOH, where B+ is an appropriate cation. Acids and bases can be grouped into two general types: strong and weak acids and bases. The difference between the two is straightforward: a strong acid in a water solution decomposes 100% into a proton (H+) and anion (A-) 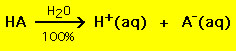 On the other hand most weak acids decompose significantly less than 100% in a water solution: 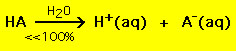 . .In other words most weak acid molecules stay intact in water. Similar chemical equations hold for strong and weak bases. There are only a few weak acids and bases, they are:
All other acids and bases are weak. A weak acid results from any anion. Examples are given below
In a typical acid/base reaction the acid and base react to form a salt and water e.g., hydrocyanic acid and sodium hydroxide: |

