|
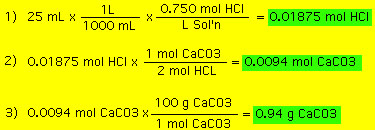
Titrations:
In a titration a known volume of a reactant (titrant),
with known concentration, is slowly added to a vessel with another
reactant until the reaction is complete. The point at which the
reaction is complete is known as the "end-point".
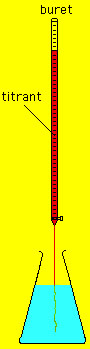
This situation is depicted in the figure on the left. Typically
the end-point is determined visually through either a color change
or formation of a precipitate. Consider the acid/base reaction:
NaOH(aq) + HCl(aq) ----> NaCl(aq) + H2O(l)
Suppose we have flask of HCl but do not know what its concentration
is. We are told that we need to know how many moles of HCl there
is in this solution. We do a titration experiment: In our laboratory
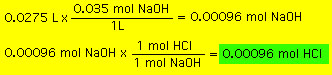
we find a bottle labeled 0.035 M NaOH. We fill a buret with this
NaOH solution and put 1 drop of phenolphthalein* indicator in
the flask with the HCl. We slowly add the NaOH to the HCl until
we notice that the solution in the flask turn a slight pink color.
The amount of NaOH added was 27.5 mL. From this information and
the balanced reaction above we can determine the moles of HCl:
*phenolphthalein is an organic compound that is colorless
in acidic solution and red in a basic solution.
|