Electrostatic attractions between water and solid ions/molecules play an important role in the solubility of solids in aqueous solutions. However, there are other factors that also play an important role. One of these factors is temperature. Temperature is a direct result of the kinetic movement of the solution molecules/ions. The higher the temperature the greater the kinetic energy of the solution molecules.
The higher the kinetic energy of water molecules, the better the chance they have of dislodging solid molecules and thereby getting them into solution. This is so because the water molecules collide with the solid and higher collision energies lead to more effective dislodgment and thus solubility. In a few instances (e.g., Li2SO4 below) the solubility of the salt will decrease with temperature. This observation does not invalidate the above explanantion but rather suggests that several competing ideas need to be taken into account to fully understand chemical processes. 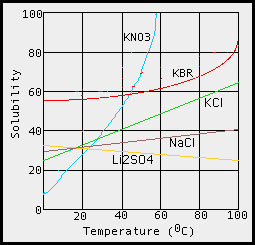
|