Parts per million (ppm) and parts per billion (ppb) are examples of expressing concentrations by mass. These units turn out to be convenient when the solute concentrations are very samll (almost trace amounts). For example, if a solution has 1 ppm solute this would mean that 1 g of solution would have one "millionth" gram of solute. Equivalently, 1 kg of this solution will have 1 mg of solute etc.. By definition we have: 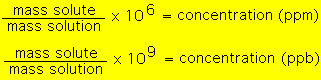 For example, suppose a 155.3 g sample of pond water is found to have 1.7x10^-4 g of phosphates. What is the concetration of phosphates in ppm?  A similar procedure would be followed to calculate ppb. In the above example the pond water would be 1,100 ppb. Now suppose we have 400 g sample of pond water and it has a concentration of 3.5 ppm dissolved nitrates. What is the mass of dissolved nitrates in this sample? 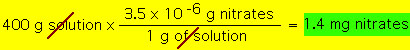 |

