Molarity is the most useful concentration for chemical reaction in solution because it directly relates moles of solute to volume of solution. The definition of molarity is As an example, suppose we dissolve 23 g of ammonium chloride (NH4Cl) in enough water to make 145 mL of solution. What is the molarity of ammonium chloride in this solution? 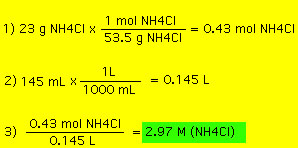 Now, suppose we have a beaker with 175 mL of a 0.55 M HCl solution. How many moles of HCl is in this beaker? As a final example suppose we have a solution of 0.135 M NaCl and we need 1.2 moles of NaCl. What volume of the NaCl solution is required? |

