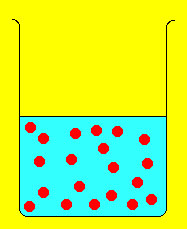
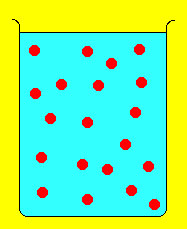
Often it is necessary to take a concentrated solution and dilute it. However, we want to dilute it in a controlled way so that we know the concentration after dilution. The way this is done can be extracted from the following figure of dilution:
The solute (denoted by red disks) is concentrated in the beaker on the left. Adding water dilutes the solution as shown with the beaker on the right. However, note that although the concentration changes upon dilution, the number of solute molecules does not. In other words the number of moles of solute is the same before and after dilution. Since Moles = Molarity x Volume (i.e., moles= M x V) we end up with the following equation relating molarity and volume before and after dilution: where i and f stand for initial and final. Suppose we need 150 mL of 0.25 M NaCl. On the shelf we find a bottle of 2M NaCl. What do we do? the concentrated molarity is Mi and the volume needed is Vi. We need to determine Vi and can do so by rearranging the above equation and doing the resulting calculation: 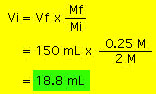 Thus, we need 18.8 mL of the 2M NaCl solution, put it in a beaker and add enough water to make 150 mL of solution. the resulting solution will have a molarity of 0.25 M NaCl. Example 2: We take 25 mL of a 0.45 M AgNO3 solution and dilute it to 300 mL. What is the molarity of the resulting solution?  |